| University | Nanyang Technological University (NTU) |
| Subject | MS2016: Phase Transformation and Kinetics |
Part 1 – Data Analysis
Plot Dilatometer vs Temperature
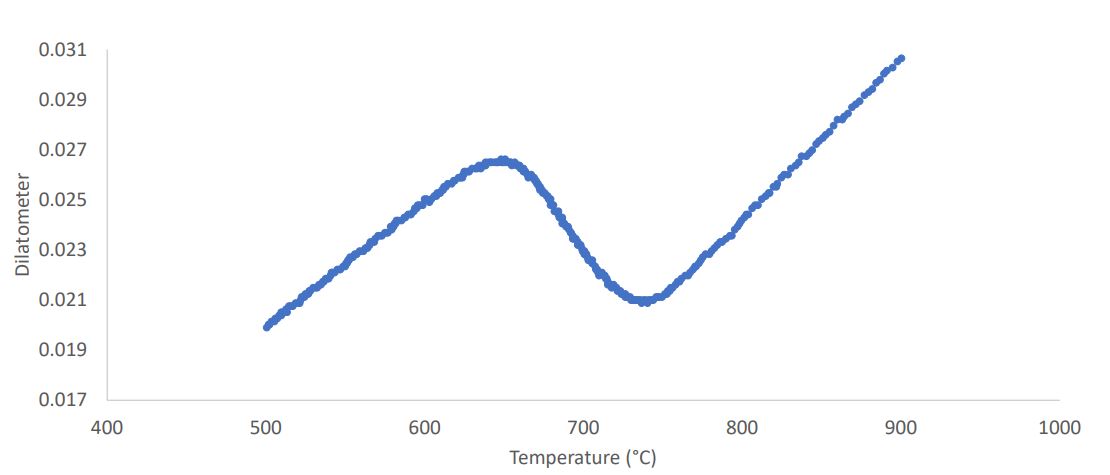
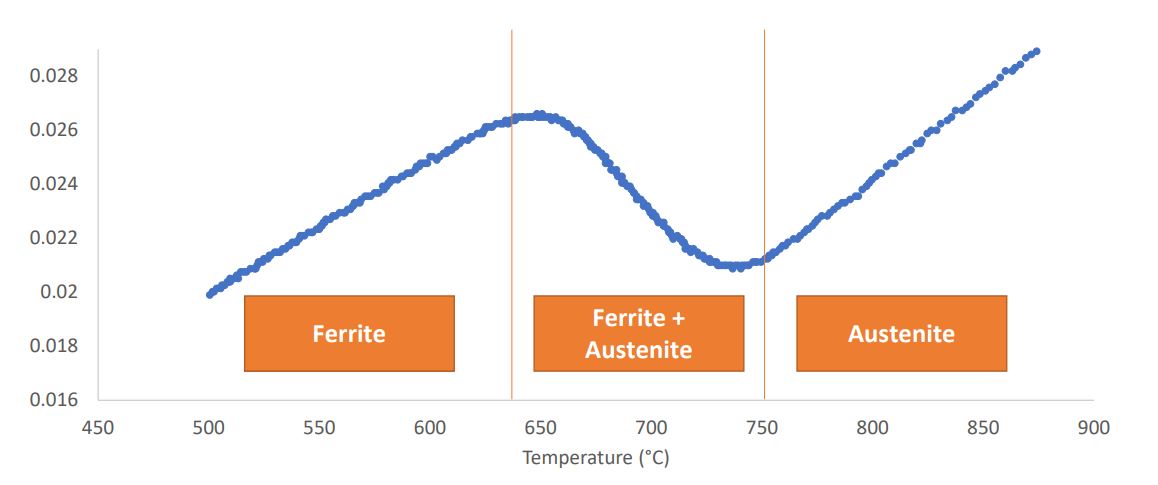
Identify 1 and 2 Phase Regions
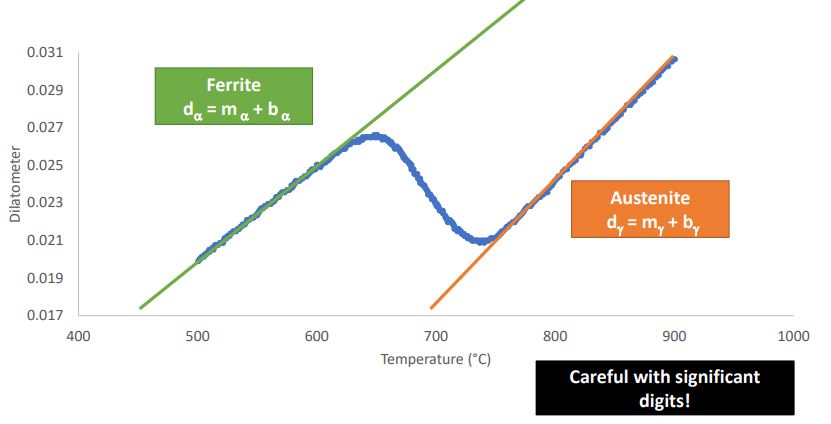
Find a Linear Equation for Each 1 Phase Field
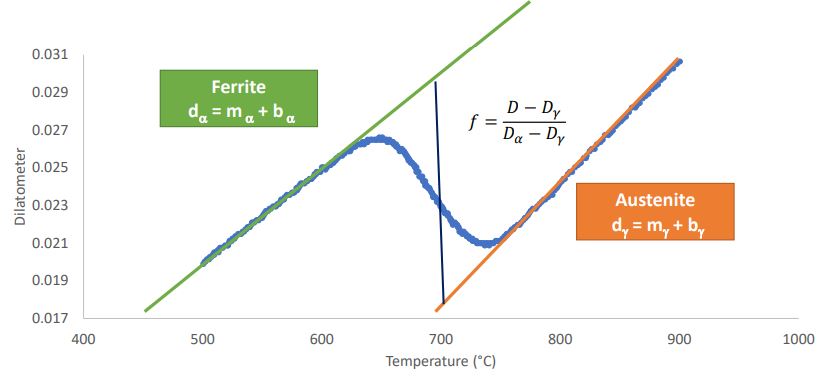
Calculate the Fraction Transformed
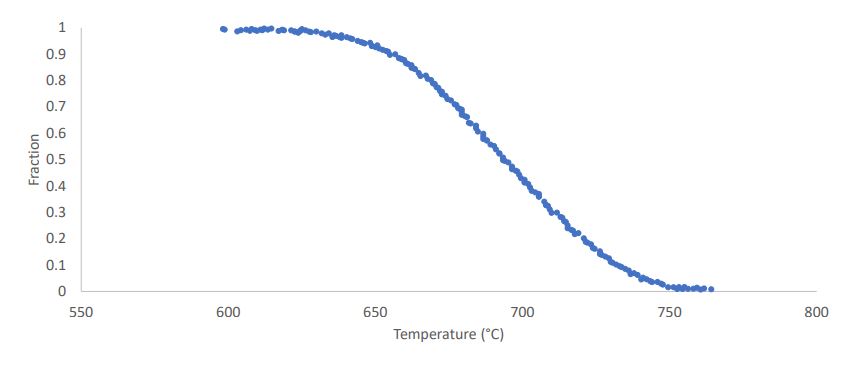
Plot Fraction Transformed vs Temperature
Plot ln(b) vs Temperature
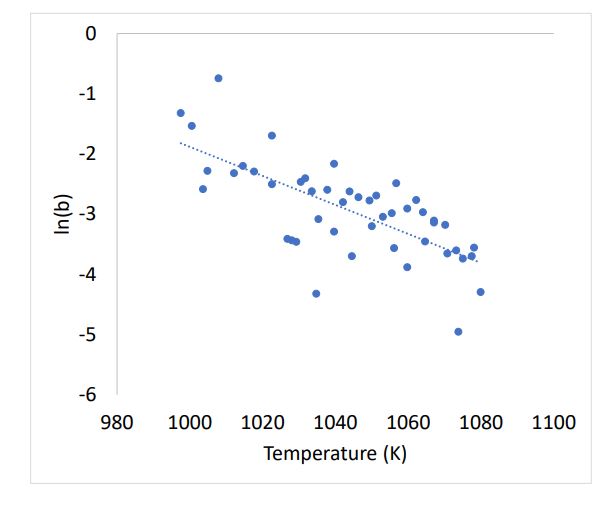
- Solve the constants for the equation: ln(b) = m T + k
- The resulting equation is the rate parameter “b” as a function of temperature.
Hire a Professional Essay & Assignment Writer for completing your Academic Assessments
Native Singapore Writers Team
- 100% Plagiarism-Free Essay
- Highest Satisfaction Rate
- Free Revision
- On-Time Delivery
Part 2: Modelling
Instructions
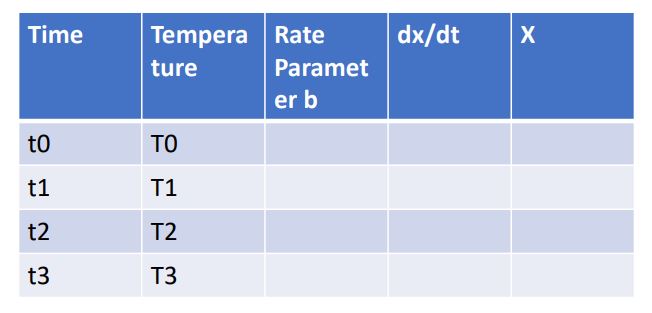
- Determine the cooling rate from the data.
- Develop a table as indicated on the left.
- The time increases with the time stamp, which you should determine as reasonable for your model. It should give you 100-200 points through the transformation range at least.
- The temperature can be calculated based on the cooling rate and the time step. The first value of temperature is where you start your model.
- The rate parameter can be calculated from the equation you found.
- dX/dt can be calculated with the rate parameter.
- The fraction transformed can be calculated as:
X1= X0 + dX/dt (t1-t0)
Get Help By Expert
We at Singapore Assignment Help with an excellent team of expert assignment writers who offer assistance for (MS2016) Phase Transformations and Kinetics assignments to NTU students. Our online assignment helper offers instant support for all kinds of assignments.
Answer
Looking for Plagiarism free Answers for your college/ university Assignments.
Recent Solved Questions
- 7WB52012 Career Research Assignment: Post-MBA Executive Role Analysis and Self-Development Planning
- ACFI3004 Australian Tax Residency & Income Assessment: Heny & Joceline Case Analysis
- CSIT213 Java OOP Assignment 1: ECommerce Management System Implementation Without Collections
- A2369C cGMP Compliance Assignment: Internal Audit CAPA Report for Quality Issues in Pharmaceutical Manufacturing
- E2419C Health Products Logistics Assignment: Cold Chain & DG Pharma Handling Case Study for Regulatory Compliance in Singapore
- AVET104 Journey Through the Cell Assignment: A Molecular Adventure into Life’s Inner Workings
- Workplace Risk-Based Assessment 1: Evaluation of Hazards, Accidents, and Safety Compliance
- SRM Reflective Assignment 2: Applying Gibbs Model to Overcome Workplace Report Challenges
- ACLP M1P TAE Written Assignment: Skills Framework & Lesson Plan Design Using Gagne’s and Kolb’s Models
- EGH222 Healthcare Analytics Assignment 2: Predictive Model for Sick Days Based on Employee Demographics and Lifestyle Data

