| University | Republic Polytechnic (RP) |
| Subject | A2369C: Current Good Manufacturing and Laboratory Practices |
Part-Time Diploma in Applied Science (Pharmaceutical Sciences)
Graded Assignment
There are THREE (3) COMPULSORY questions for this graded assignment. You must adhere to the word limits indicated for the question as required. Words more than the stipulated word limit will not be taken into consideration even if the correct answer is contained therein.
Question 1 (Total: 24 marks)
Republic Pharma company is commissioning a new manufacturing line that is based on the picture below. As part of the project team, you are tasked to contribute to the planning and documentation strategy required.
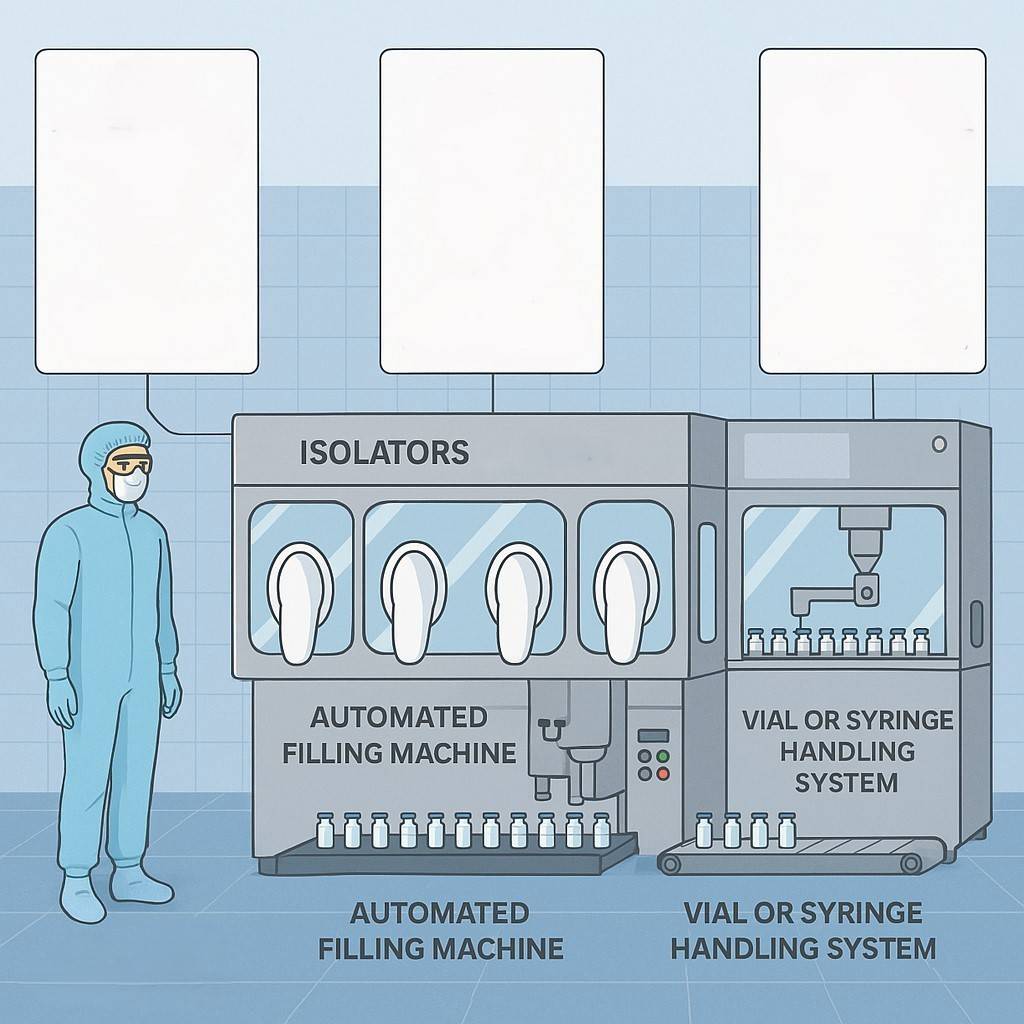
a) Identify and describe three (3) critical GMP documents needed before production can begin, explaining their purpose and key elements. (6 marks)
b) Outline the steps required to validate the production line (IQ, OQ, PQ) and describe the importance of each step. (6 marks)
c) Discuss the regulatory considerations (e.g., audits, authority approvals, registration) that must be fulfilled before commercial release. (6 marks)
d) Propose how Good Documentation Practices (GDP) will be implemented across the team to ensure traceability and compliance. (6 marks)
Question 2 (Total: 20 marks)
a) Identify and explain four (4) essential components of Good Laboratory Practice (GLP) in the context of the picture below. (8 marks)
b) Describe the role of a Quality Assurance Unit (QAU) in GLP compliance. (4 marks)
c) Create a one-page SOP outline (in bullet format) on how to maintain a laboratory notebook in line with GLP and ALCOA+ principles. Include the Title and Purpose of the SOP. (8 marks)

Question 3 (Total: 20 marks)
A research associate in Republic Pharma company published promising results about a novel drug’s efficacy.
Efficacy Data for Novel Drug “NeuroVex” Study Design:
- Randomized, double-blind, placebo-controlled trial
- Sample size: 120 patients (60 drug, 60 placebo)
- Indication: Treatment of early-stage neurodegenerative disorder
- Duration: 12 weeks
Primary Endpoint: Improvement in cognitive function score (CF Score)
Internal results recorded on laboratory notebook:
| Group | Baseline CF Score | Week 12 CF Score | Mean Change |
| NeuroVex | 45.2 | 55.0 | +9.8 |
| Placebo | 44.9 | 48.1 | +3.2 |
Results shown on published scientific article:
| Group | Baseline CF Score | Week 12 CF Score | Mean Change |
| NeuroVex | 45.2 | 62.8 | +17.6 |
| Placebo | 44.9 | 48.1 | +3.2 |
Task
a) Compare the published results with the internal lab data. Identify and evaluate the ethical breaches that may have occurred based on the discrepancies you observe. (6 marks)
b) Discuss the potential regulatory and legal implications for the company and the researcher if these discrepancies are verified during an inspection or audit. (6 marks)
c) Propose a three-step action plan the company should take to investigate, address the situation, and prevent recurrence of such an incident. (8 marks)
Use diagrams, tables or flowcharts where relevant to support your report. (3 marks) Cite at least three references to support your recommendations. (3 marks)
(Word limit: 2000 words)
Hire a Professional Essay & Assignment Writer for completing your Academic Assessments
Native Singapore Writers Team
- 100% Plagiarism-Free Essay
- Highest Satisfaction Rate
- Free Revision
- On-Time Delivery
Do you require professional help with your pharmaceutical assignments? Professional assistance on GMP, GLP, and ethical matters is provided by SG Assignment Help. Our knowledgeable staff guarantees excellent work, whether it's compliance reports or in-depth analysis. Obtain trustworthy report writing help that is suited to your academic requirements. For professionally written assignments that will improve your grades, get in touch with SG Assignment Help right now!
Looking for Plagiarism free Answers for your college/ university Assignments.
- GSS503 Navigating Risk in an Interconnected World Course Tutor-Marked Assignment 01, 2026
- GSS501 Global Crime Prevention and Security Management Tutor-Marked Assignment 01, 2026
- PSB6012CL Business Research Methods Assignment Brief 2026 | Coventry University
- MTH109 Calculus Tutor-Marked Assignment 1, 2026 | SUSS
- BUS286 Corporate Finance Assignment 2026 | Murdoch University
- HFSY359 Fatigue Management Tutor Mark Assignment Question 2026 | SUSS
- BSE313 Sport Coaching Tutor-Marked Assignment 2 Question 2026 | SUSS
- 6079MP Final Coursework Assignment 2026 | Coventry University
- IBUS2004 Managing International Business Assessment 1 Brief 2026 | UON
- BSL305 Company Law Assignment 2026 | Murdoch University

